ISO 9001 versus ISO 13485 – What are the differences and which certification is best adapted to medical devices manufacturers?
Developing products with highest quality standards is essential for medical device manufacturers. Having been certified to both ISO 9001 and ISO 13485 for the past decade, Valtronic and quality manufacturing are long time companions. However, is it still necessary to keep both certifications when dedicated to the medical industry? Let’s briefly review the two standards and explain Valtronic’s decision to embrace essentially ISO 13485.
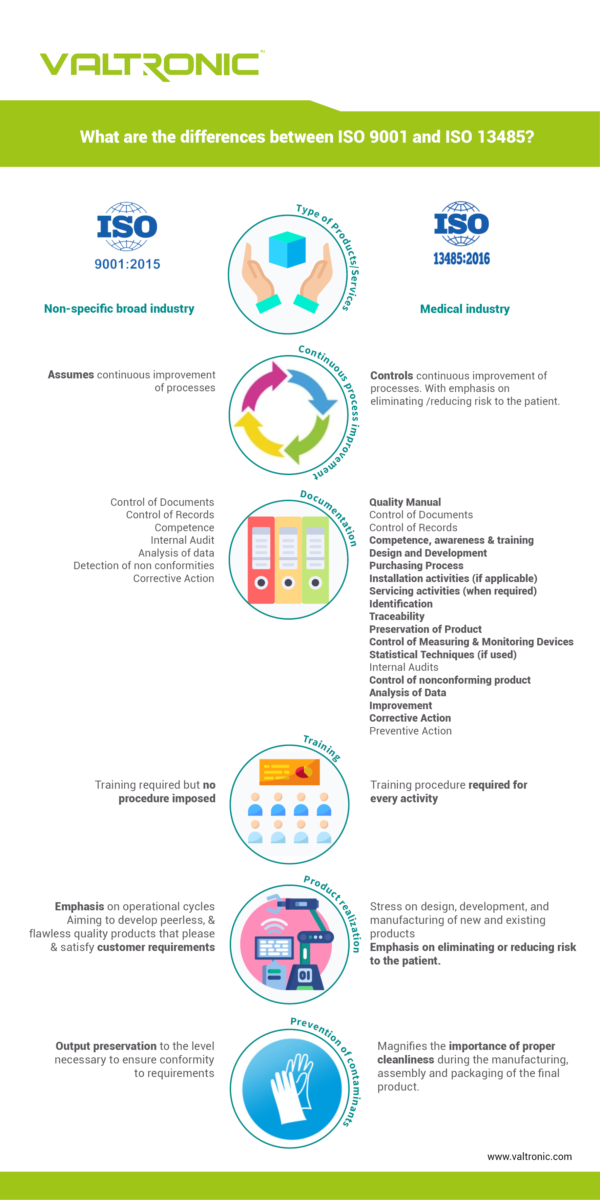
What are the differences between the two certifications?
The emphasis of ISO 9001 is on continuous improvement and customer satisfaction in the manufacturing and service industries with minimal regulatory compliance focus. ISO 9001 specifies the guidelines to organize a Quality Management System (QMS) and its effective implementation. It was designed to sufficiently meet all the mandatory needs and demands of the customer and set the expectation for constant quality improvement. It is used by a wide variety of non-regulated industries.
The medical device industry is of course also customer oriented and the continuous improvement of its processes is key to provide products with highest quality standards. However, regulatory compliance is the first and highest priority of ISO 13485 in developing and manufacturing medical devices toward mitigating human safety. That is why the ISO 13485 system was developed. ISO 9001 was used as a starting point, but ISO 13485 was specifically developed for medical device related industries and their quality management systems (QMS). It focuses on the development and manufacturing of a medical device, as well as on the management of the device’s whole lifecycle and traceability. ISO 13485 was developed to ensure reliability, quality, trust and strong commitment to human safety.
In a nutshell, ISO 9001 is more customer oriented whereas ISO 13485 is more product oriented. (Have a look at our infographics for more details about the differences existing between the two systems).
What about Valtronic – does it make sense to keep both certification systems?
Valtronic is dedicated to developing, industrializing, and manufacturing medical devices at the highest quality standards for our customers and the patients who will benefit from these innovative technologies. The ISO 13485 certification is therefore essential to our core activities. For the past decade, Valtronic had maintained both certifications for all sites. However became apparent to us that even our non-medical customers no longer desire the ISO 9001 “general” approach to quality, recognizing the benefits of the more demanding QMS used for the medical industry. Valtronic has therefore decided not to maintain the ISO 9001 certification for our sites in Switzerland and the USA. However, both certifications will still be maintained for the Moroccan manufacturing site as their customer base includes a larger share of select industrial customers as well as those in the medical device industry.
Article written in collaboration with : Mark Zwolinski – Quality Manager & ISO Management Representative
ISO 9001 vs ISO 13485 Infographics

