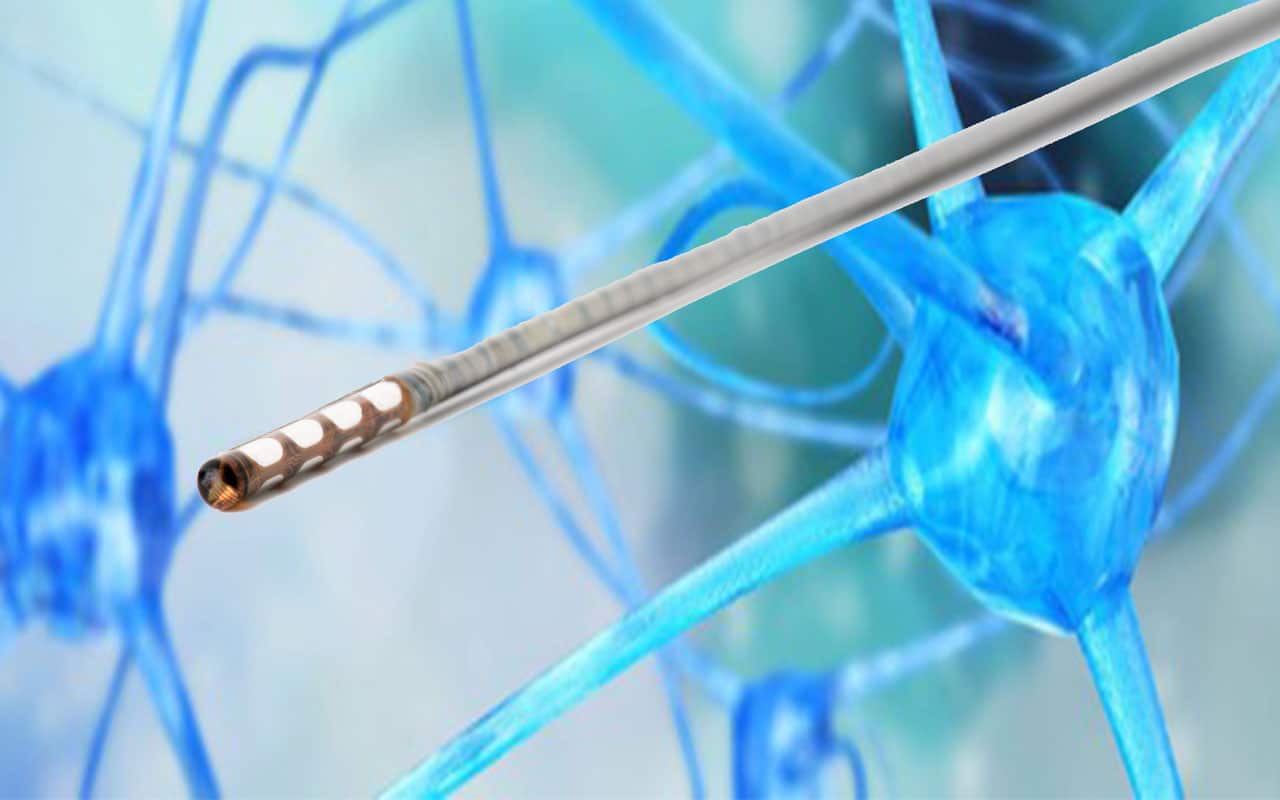
Aleva Neurotherapeutics SA is developing the next generation of implantable Directional Deep Brain Stimulation (DBS) system. These implants help patients with Parkinson’s disease control symptoms such as tremor. Aleva’s Neurotherapeutics directSTIM™ system is unique as its directional stimulation allows the area of the brain to be treated with targeted precision. This will improve therapeutic response by reducing the risk of side effects. Aleva chose Valtronic to industrialize and manufacture their innovative DBS lead based on MEMS thin-film technology.
MEMS thin-film technology requiring a customized clean room assembly process
The directSTIM™ DBS lead is extremely thin. With a diameter of 1.3 mm and a length of 400 mm, high-precision manual assembly is required. Starting from Aleva Neurotherapeutics’ proof of concept, Valtronic developed a customized process to assemble the lead, attach the MEMS thin film and connect the conductors to the electrodes in a reliable manner. 90% of operations are manual and performed in Valtronic’s clean room. A specific process was also developed for the device’s micro-encapsulation. “Valtronic’s experience in DFM, industrialization and manufacturing of medical devices is a real asset. They developed an assembly process with manufacturability in mind allowing for a smooth transition to the manufacturing phase”, underlines Fabrice Jendly Operations Director of Aleva Neurotherapeutics.
“Valtronic is a partner in the true sense of the word. They provide all the microelectronic expertise required for the industrialization and manufacturing of our DBS probe. On top of this, we benefit from high reactivity, open communication and geographic proximity which allows for highly efficient project management”.
Fabrice Jendly, Operations Director at Aleva Neurotherapeutics SA
The directSTIM™ DBS System used in Parkinson’s disease
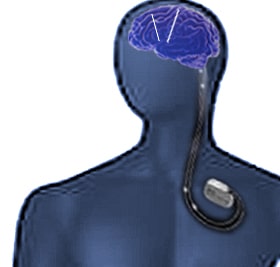
DBS involves implanting programmable multi-contact electrodes in specific anatomic targets “deep” within the brain. These electrodes are connected by an extension wire that connects to a neurostimulator typically placed subcutaneously below the clavicle. DBS is used to treat a variety of disabling neurological symptoms resulting from Parkinson’s disease, such as tremor, rigidity, stiffness, and slowed movements. DBS uses a surgically implanted, battery-operated medical device called a neurostimulator to deliver electrical stimulation to targeted areas in the brain that control movement. Compared to current DBS systems, Aleva Neurotherapeutics innovates with directional stimulation. directSTIM™ uses an array of directional microelectrodes, which allows it to focus on the electrical stimulation. This allows for improved therapeutic response by reducing the risk of side effects. Also, the implant requires less time for surgical implantation and can be left in place as a permanent implant. Patients will benefit from this system after obtaining the CE Mark, that is expected in the second half of 2019.
More about Aleva Neurotherapeutcis at: www.aleva-neuro.com